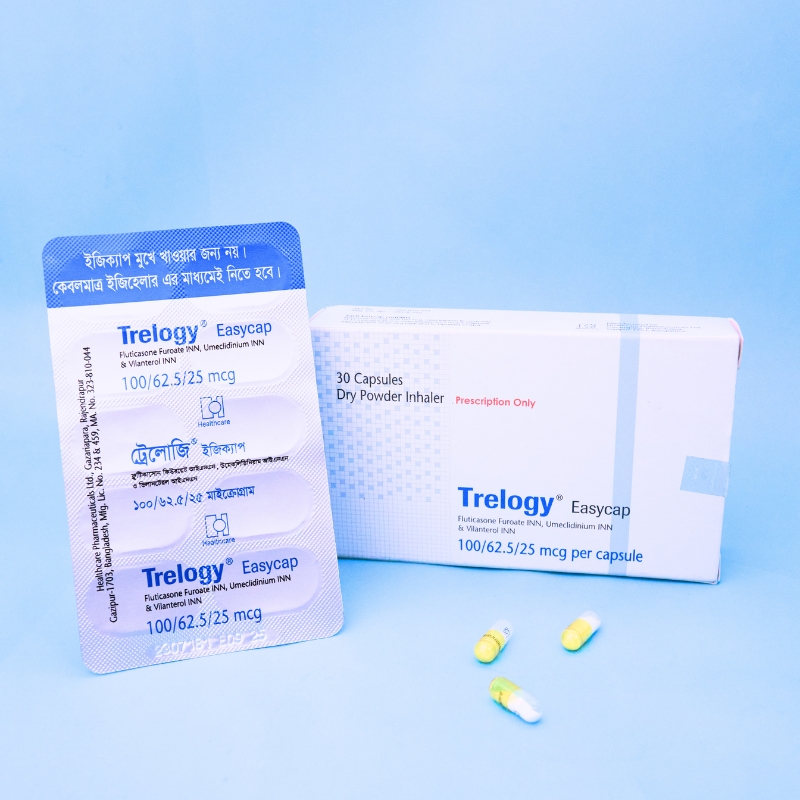
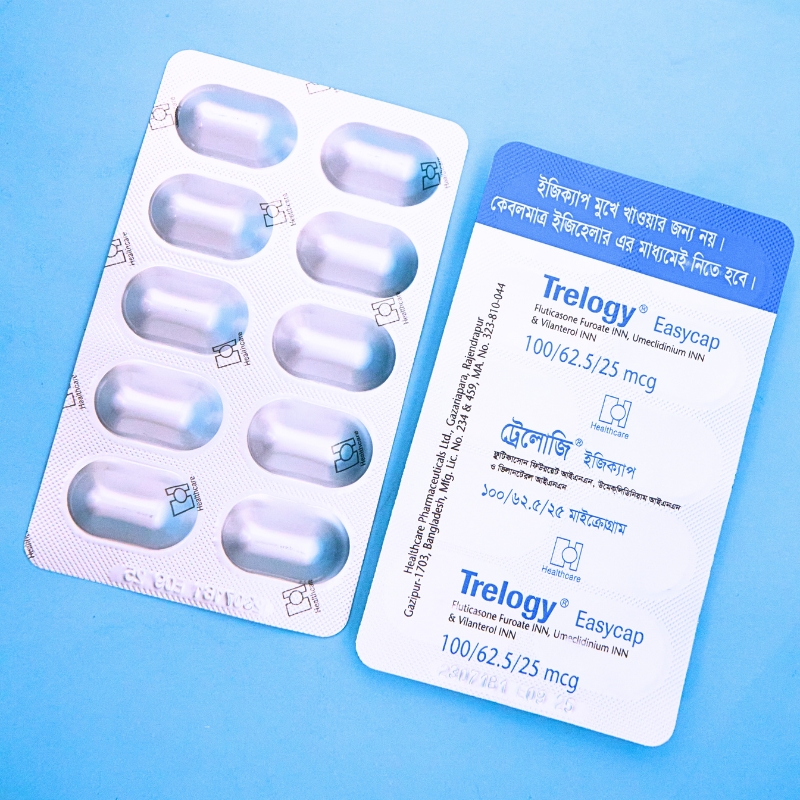
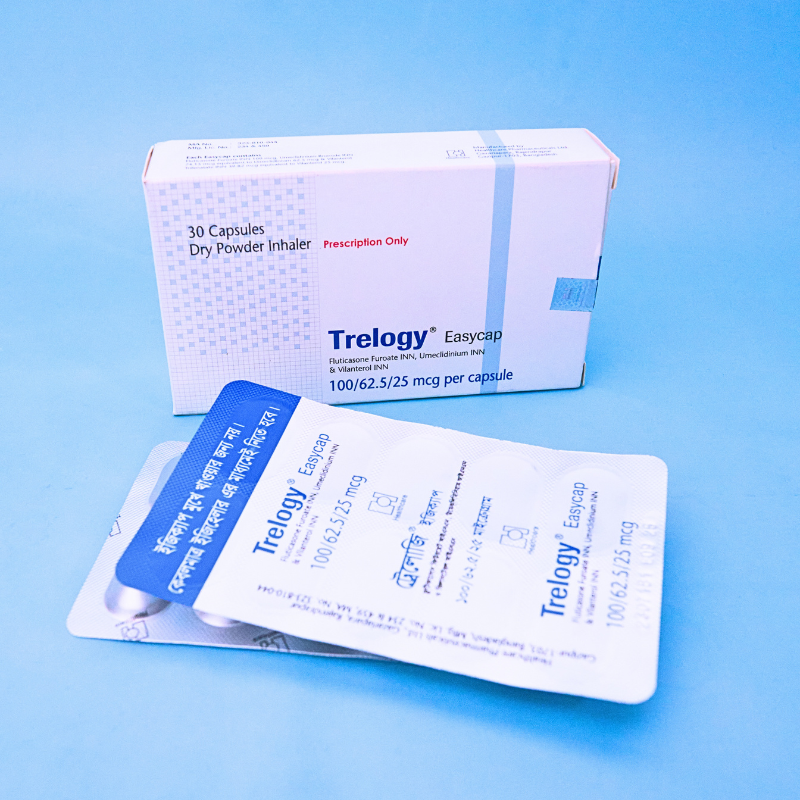
Trelogy DPI is indicated for:
Maintenance treatment of Chronic Obstructive Pulmonary Disease (COPD)
Maintenance treatment of Asthma in patients aged 18 years and older
❗ Limitation of Use: Not intended for relief of acute bronchospasm
Each dry powder capsule contains:
Vilanterol 25 mcg (as Vilanterol Trifenatate INN)
Fluticasone Furoate 100 mcg or 200 mcg (INN)
Umeclidinium 62.5 mcg (as Umeclidinium Bromide INN)
Trelogy combines three active components:
Vilanterol (a long-acting beta2-agonist or LABA) relaxes airway muscles by increasing cAMP levels.
Fluticasone Furoate (an inhaled corticosteroid or ICS) reduces airway inflammation involved in asthma and COPD.
Umeclidinium (a long-acting muscarinic antagonist or anticholinergic) blocks M3 receptors, leading to bronchodilation.
Together, these agents improve lung function and control chronic respiratory conditions.
This capsule is for inhalation only — do not swallow.
Use with the prescribed inhaler device. Remove capsule from blister only before use.
Rinse mouth with water (do not swallow) after inhalation to avoid oral candidiasis.
Adults (18+):
COPD: 1 inhalation capsule once daily
Asthma: 1 inhalation capsule once daily
Use at the same time every day, not more than once in 24 hours
If shortness of breath occurs between doses, use a Short-Acting Beta-Agonist (SABA) for immediate relief
CYP3A4 inhibitors (e.g., ketoconazole): May increase systemic corticosteroid effects
MAOIs / Tricyclic antidepressants: May enhance vilanterol’s cardiovascular effects
Beta-blockers: May reduce effectiveness and worsen bronchospasm
Diuretics: Risk of ECG changes and hypokalemia
Other anticholinergics: Avoid co-administration due to additive effect
Acute management of status asthmaticus or severe COPD/asthma
Known hypersensitivity to milk proteins or any component of the product
COPD Patients (≥1% incidence):
URTI, pneumonia, bronchitis, oral thrush, headache, back pain, influenza, sinusitis, pharyngitis, constipation, UTI, diarrhea, cough, dysphonia
Asthma Patients (≥2% incidence):
Pharyngitis, viral RTI, bronchitis, sinusitis, rhinitis, UTI, headache, back pain
Safety data in pregnant or breastfeeding women are insufficient
Use only if clearly needed and under medical supervision
LABA monotherapy increases risk of asthma-related death
Not for acute asthma or COPD attacks
Avoid use with other LABAs
Risk of oral candidiasis – rinse mouth after use
Monitor for pneumonia in COPD patients
Discontinue if paradoxical bronchospasm occurs
Use cautiously in patients with heart disease, urinary retention, diabetes, seizures, hyperthyroidism
Children & Adolescents (<18 years): Not recommended
Elderly: No dose adjustment required, but monitor closely
Renal or Hepatic Impairment: Not studied; use with caution
No clinical trial data available on overdose
Symptoms may include tremor, headache, increased heart rate
Store in a cool, dry place
Avoid direct sunlight and heat
Keep out of reach of children
Keep away from eyes