For adults aged eighteen years and older, the recommended dose is one inhalation daily, taken at the same time each day, with no more than one dose in twenty-four hours. It is not intended for use in children or adolescents. Elderly patients may take the usual dose. Patients with mild to moderate kidney or liver problems can use the standard dose, but those with severe impairment should only use it if the expected benefit outweighs the risk.
Remove the protective cap from the inhaler and open it by turning the mouthpiece as directed. Take a capsule from its blister, place it in the chamber, and close the mouthpiece. Hold the inhaler upright, press both side buttons once to pierce the capsule, then release them. Breathe out fully away from the inhaler, tilt your head slightly back, place the mouthpiece in your mouth, and inhale quickly and deeply. A whirring sound indicates the powder is being released. Hold your breath for five to ten seconds, then remove the empty capsule and rinse your mouth. Clean the inhaler after use by washing and drying the mouthpiece, brushing the chamber, and clearing the piercing needles before replacing the cap.
No dedicated interaction studies have been conducted, but the medicine should not be used with beta-blockers, other anticholinergics, or sympathomimetic drugs, as side effects may increase. Caution is needed if used with medicines that lower potassium levels.
This medicine should not be used by individuals allergic to Indacaterol, Glycopyrronium, any other ingredient in the formulation, or milk proteins. It is also not for asthma patients unless combined with an appropriate asthma control treatment.
Common reactions include cough and throat irritation. Possible effects from the muscarinic antagonist component include dry mouth, blurred vision, constipation, urinary retention, and gastrointestinal discomfort. Effects from the beta2-agonist component may include palpitations, tremor, headache, muscle cramps, changes in heart rate or blood pressure, low potassium, and high blood sugar. Serious allergic reactions, chest pain, or worsening breathing should receive immediate medical attention.
Classified as pregnancy category C, this medicine should only be used in pregnancy if the potential benefit outweighs the risk to the fetus. It is unknown if the drug passes into breast milk, so its use during breastfeeding should also weigh benefits against risks.
It should not be started in cases of sudden worsening of breathing and should not be combined with other long-acting beta2-agonists to avoid overdose. If breathing worsens after use, discontinue immediately. Use with caution in patients with cardiovascular disease, thyroid disorders, seizures, diabetes, glaucoma, or urinary problems, as these conditions may worsen. Blood sugar and potassium levels should be monitored when necessary.
Taking too much may cause exaggerated beta2-agonist effects such as tremor, rapid heartbeat, headache, nausea, vomiting, drowsiness, vision changes, urinary difficulty, or increased eye pressure. Supportive treatment and hospital monitoring may be needed in severe cases.
Keep away from direct sunlight and heat, store in a cool, dry place, and ensure it is out of the reach of children.
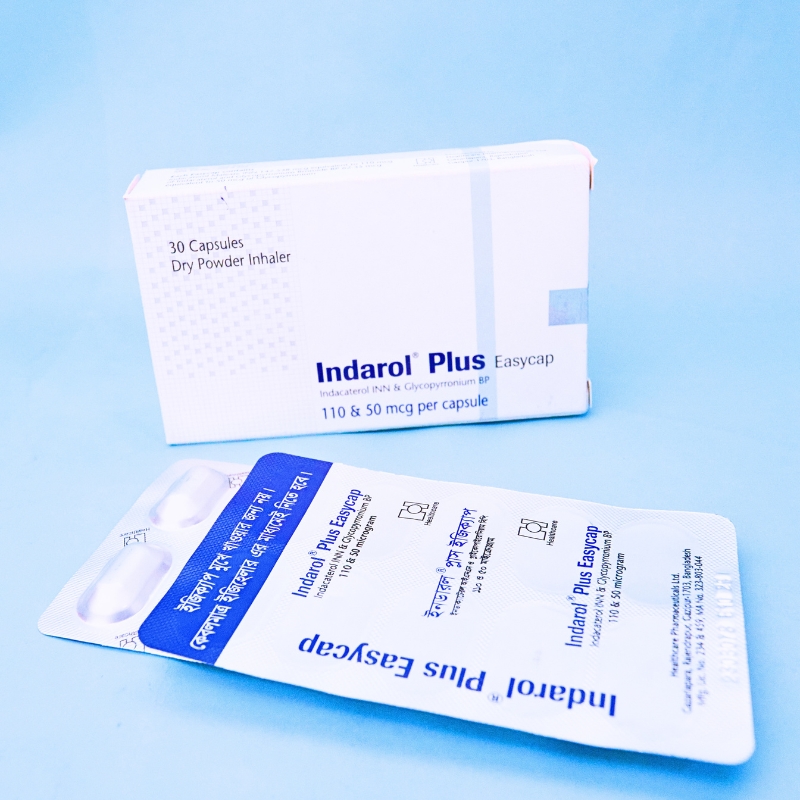
Each capsule for inhalation contains Indacaterol 110 micrograms as Indacaterol Maleate and Glycopyrronium 50 micrograms as Glycopyrronium Bromide.
This inhalation powder combines Indacaterol, a long-acting beta2-adrenergic agonist, and Glycopyrronium, a long-acting muscarinic antagonist. Indacaterol works by stimulating beta2 receptors in the lungs, increasing cyclic AMP levels, relaxing airway muscles, and reducing the release of inflammatory chemicals. Glycopyrronium blocks M3 receptors in the airway smooth muscles, which further relaxes the airways and improves airflow.

.jpg)
.jpg)
.jpg)
.jpg)