Montelukast controls asthma by preventing leukotriene activity.
Montelukast is indicated for the prophylaxis and chronic treatment of asthma, the prevention of exercise-induced bronchoconstriction, and the relief of symptoms of allergic rhinitis in adults and pediatric patients.
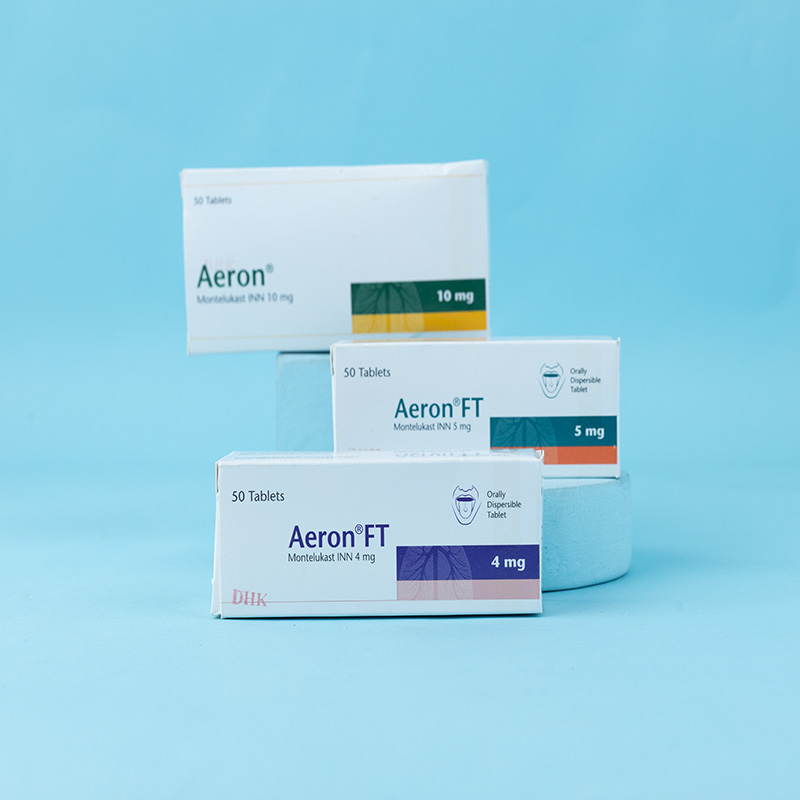
Adults (15 years of age or older): One 10 mg tablet daily in the evening.
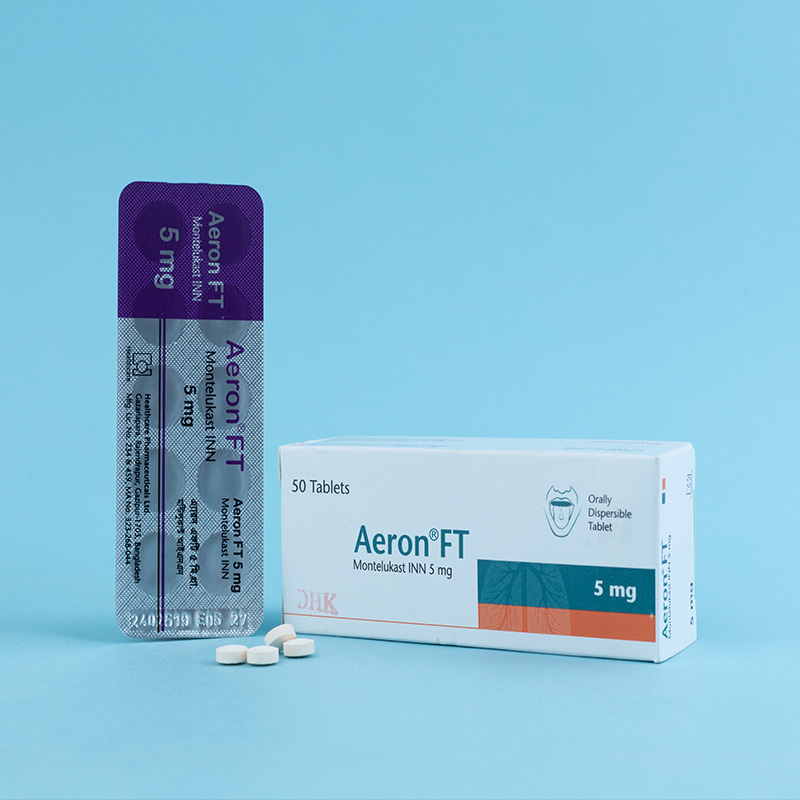
Children (6-14 years of age): One 5 mg tablet daily in the evening.
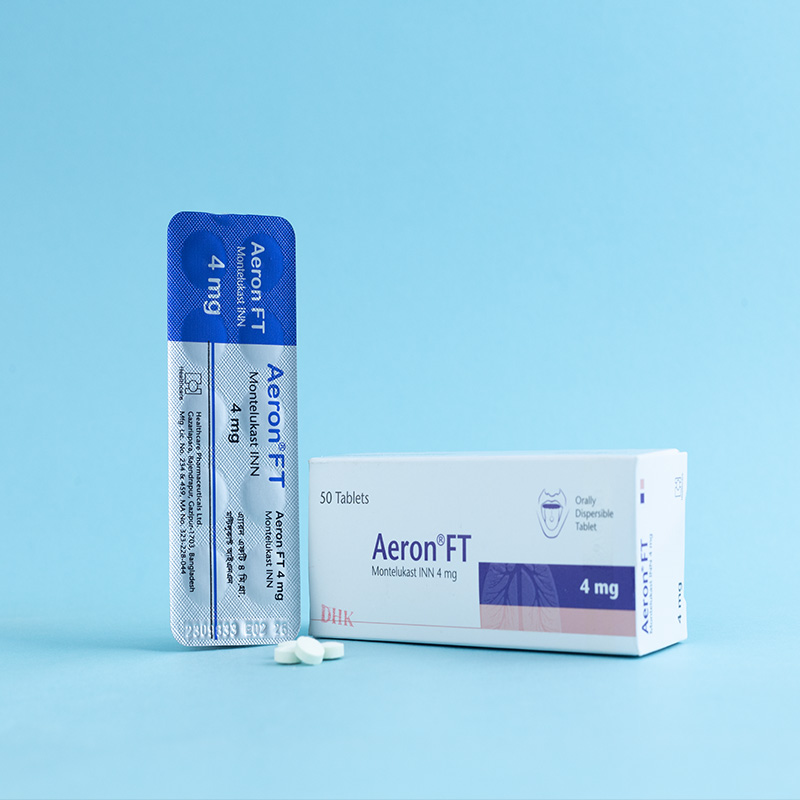
Children (6 months-5 years of age): One 4 mg orally dispersible tablet daily in the evening.
Montelukast is generally well-tolerated. Side effects may include dizziness, headache, diarrhea, restlessness, abdominal pain, cough, fever, asthenia, rash, and upper respiratory tract infection.
Montelukast is contraindicated in patients with hypersensitivity to any component of this product.
Montelukast is not indicated for reversing bronchospasm in acute asthma attacks (e.g., status asthmaticus).
Patients with known aspirin sensitivity should continue to avoid aspirin or other NSAIDs while taking Montelukast.
There are no adequate data on the use of Montelukast in pregnant women or lactating mothers.
Montelukast has been administered with other therapies routinely used for the prophylaxis and chronic treatment of asthma without any significant increase in adverse reactions.
There have been no serious adverse experiences reported in most cases of overdose. The most frequently observed symptoms include thirst, mydriasis, hyperkinesia, and abdominal pain. In the event of overdose, standard supportive measures should be employed, such as removing unabsorbed material from the gastrointestinal tract, clinical monitoring, and supportive therapy if required.
Store in a cool, dry place below 30°C. Protect from light and moisture.
Medicine: Keep out of reach of children.
Pharmacodynamic Properties: Montelukast is a selective leukotriene receptor antagonist (LTRA) that blocks the action of cysteinyl leukotrienes (CysLTs) at the CysLT1 receptor. CysLTs such as LTC₄, LTD₄, and LTE₄ are inflammatory mediators released by mast cells and eosinophils. They are potent bronchoconstrictors and contribute to airway edema, smooth muscle contraction, and altered cellular activity associated with the inflammatory process in asthma and allergic rhinitis.
Montelukast inhibits bronchoconstriction induced by allergens and exercise, reduces eosinophilic inflammation, and improves asthma control, especially in patients with aspirin-sensitive asthma.
Pharmacokinetic Properties:
Absorption: Montelukast is rapidly absorbed following oral administration. The mean peak plasma concentration (Cmax) is achieved in 2–4 hours. The oral bioavailability is ~64% for the 10 mg tablet and ~73% for the 5 mg chewable tablet.
Distribution: Montelukast is more than 99% bound to plasma proteins. The volume of distribution at steady state is approximately 8–11 liters.
Metabolism: Montelukast is extensively metabolized in the liver by cytochrome P450 enzymes, primarily CYP3A4, CYP2C8, and CYP2C9. However, at therapeutic doses, the systemic exposure of metabolites is minimal.
Elimination: Montelukast and its metabolites are primarily excreted via the bile. The elimination half-life ranges from 2.7 to 5.5 hours in healthy adults. The pharmacokinetics are linear over the dose range studied (10 to 50 mg).
Mechanism of Action: Montelukast binds with high affinity and selectivity to the CysLT1 receptor and inhibits the physiological actions of LTD₄ without agonist activity. It thereby prevents airway inflammation, bronchoconstriction, and symptoms associated with asthma and allergic rhinitis.