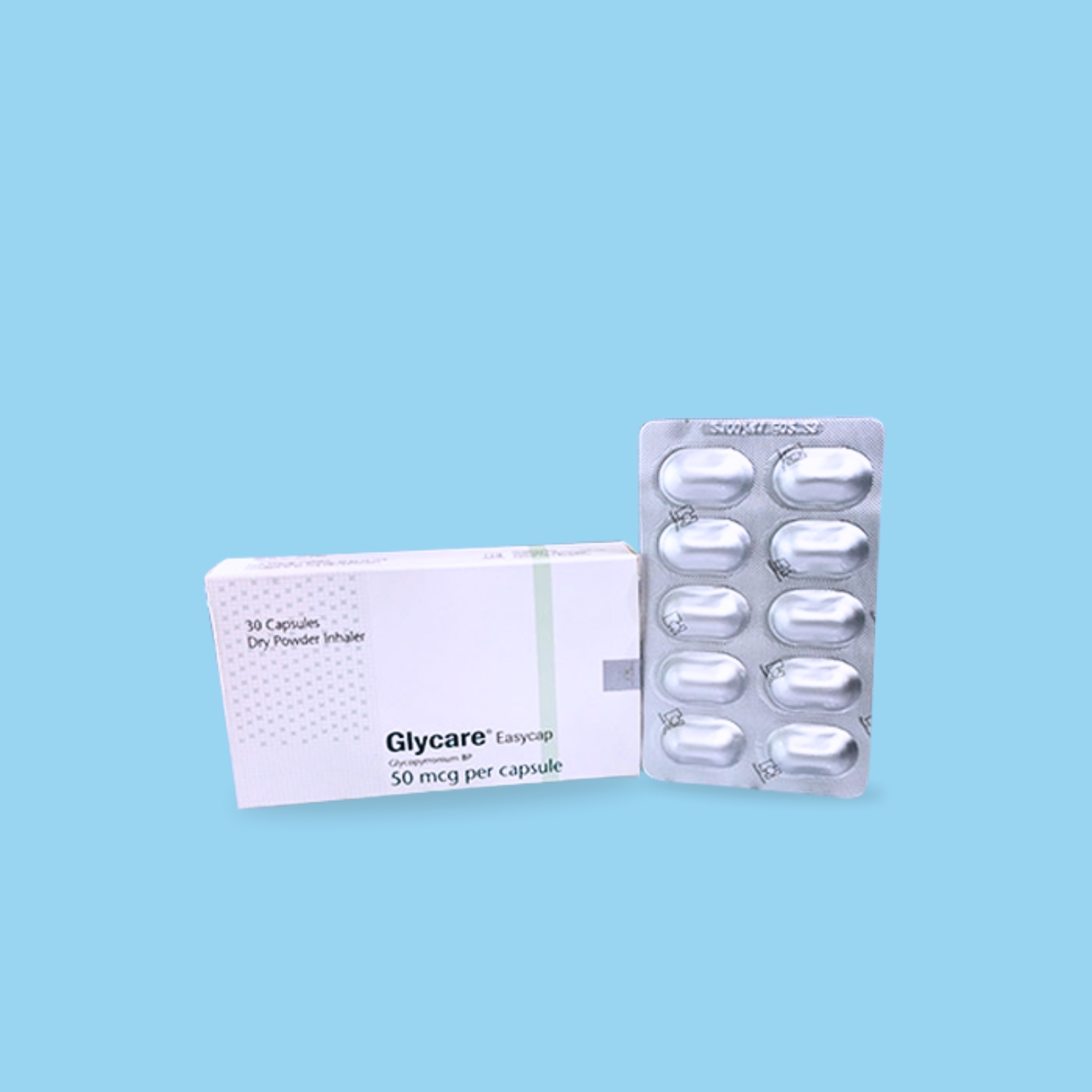
Glycare inhalation capsule is indicated as a once-daily maintenance bronchodilator treatment to relieve symptoms in patients with chronic obstructive pulmonary disease (COPD).
Glycopyrronium is a long-acting, specific antimuscarinic agent, often referred to as an anticholinergic in clinical medicine. It has a similar affinity for muscarinic receptor subtypes M1 to M5. In the airways, inhibition of M3 receptors in the smooth muscle results in relaxation. Its high potency and slow receptor dissociation contribute to significant and long-acting bronchodilation in patients with COPD.
The recommended dose is one inhalation capsule (50 micrograms) once daily using the prescribed inhalation device. This should be administered at the same time every day via the orally inhaled route. If a dose is missed, the next dose should be taken as soon as it is remembered. Do not take more than one dose in 24 hours.
No specific interaction studies have been conducted for the co-administration of this inhalation capsule with other inhaled anticholinergic drugs, and therefore, its use alongside other anticholinergics is not recommended. Although no formal drug interaction studies have been performed, this inhalation capsule has been used with other COPD medications without clinical evidence of drug interactions. These include sympathomimetic bronchodilators, methylxanthines, and oral or inhaled steroids. Concomitant administration of this inhalation capsule with orally inhaled indacaterol, a β2-adrenergic agonist, under steady-state conditions, does not affect the pharmacokinetics of either drug.
This medication is contraindicated in patients with hypersensitivity to glycopyrronium or any of the excipients in the formulation.
Common side effects include dry mouth, cough, nasopharyngitis, vomiting, musculoskeletal pain, neck pain, diabetes mellitus, gastroenteritis, and insomnia. Some less common side effects may include paradoxical bronchospasm, dysphonia, angioedema, hypersensitivity reactions, pruritus, sinus congestion, and throat irritation. In elderly patients above 75 years of age, urinary tract infections and headaches are more frequently reported.
Pregnancy: There are no adequate or well-controlled studies on the use of this inhalation capsule in pregnant women with COPD. Therefore, it should only be used if the expected benefits outweigh the potential risks to the fetus.
Lactation: It is unknown whether glycopyrronium bromide and its metabolites are excreted in human milk. Therefore, its use in breastfeeding women should be approached with caution and only if the potential benefit outweighs the possible risk to the infant.
Fertility: Animal studies do not indicate any concerns regarding fertility in either males or females.
This inhalation capsule is intended as a once-daily, long-term maintenance treatment and is not suitable for acute bronchospasm (rescue therapy).
Not recommended for patients under 18 years of age.
Like other anticholinergic drugs, it should be used with caution in patients with narrow-angle glaucoma or urinary retention.
If signs of an allergic reaction occur, particularly angioedema (difficulty breathing or swallowing, swelling of the tongue, lips, and face), urticaria, or skin rash, this medication should be discontinued immediately, and alternative therapy should be considered.
As with other inhalation therapies, administration may cause paradoxical bronchospasm, which can be life-threatening. If this occurs, treatment should be stopped immediately, and an alternative therapy should be used.
In patients with severe renal impairment (glomerular filtration rate below 30 mL/min/1.73 m²), including those with end-stage renal disease requiring dialysis, this inhalation capsule should only be used if the expected benefits outweigh the potential risks.
High doses of Glycare may lead to anticholinergic side effects, requiring symptomatic treatment. In COPD patients, repeated oral inhalation of Glycare at doses of 100 to 200 micrograms once daily for 28 days has been well tolerated. Acute intoxication due to accidental oral ingestion of Glycare capsules is considered unlikely.
This capsule must not be swallowed and should only be used with an inhalation device.
Use Easycap in the Easyhaler for the best performance.
Remove the capsule from the blister pack immediately before use. Capsules exposed to moisture may not tear easily.
Store at a temperature not exceeding 30°C in a dry place.
Protect from light and moisture.
Keep out of the reach of children.