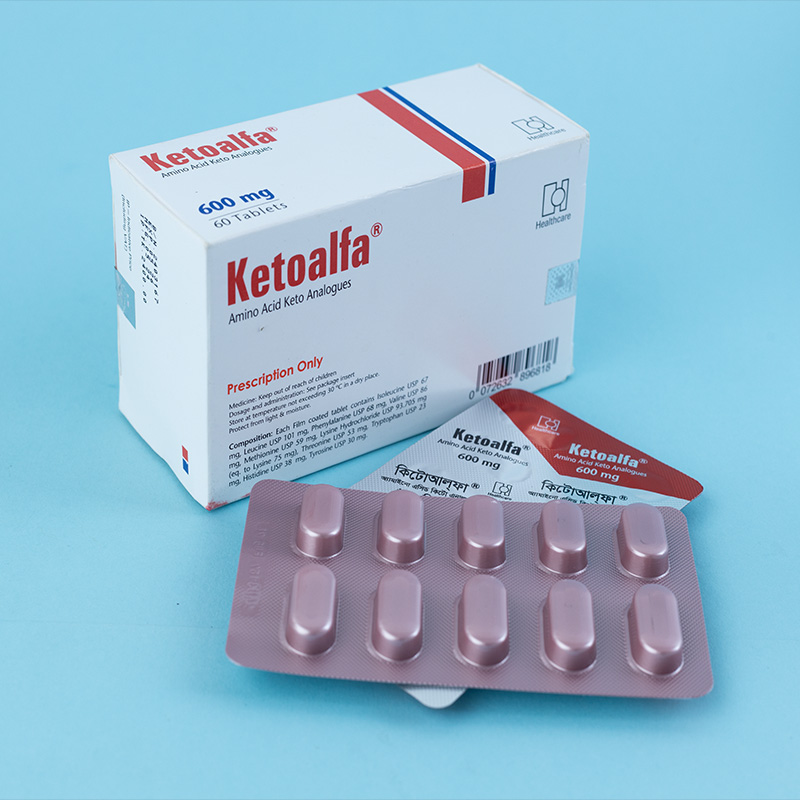
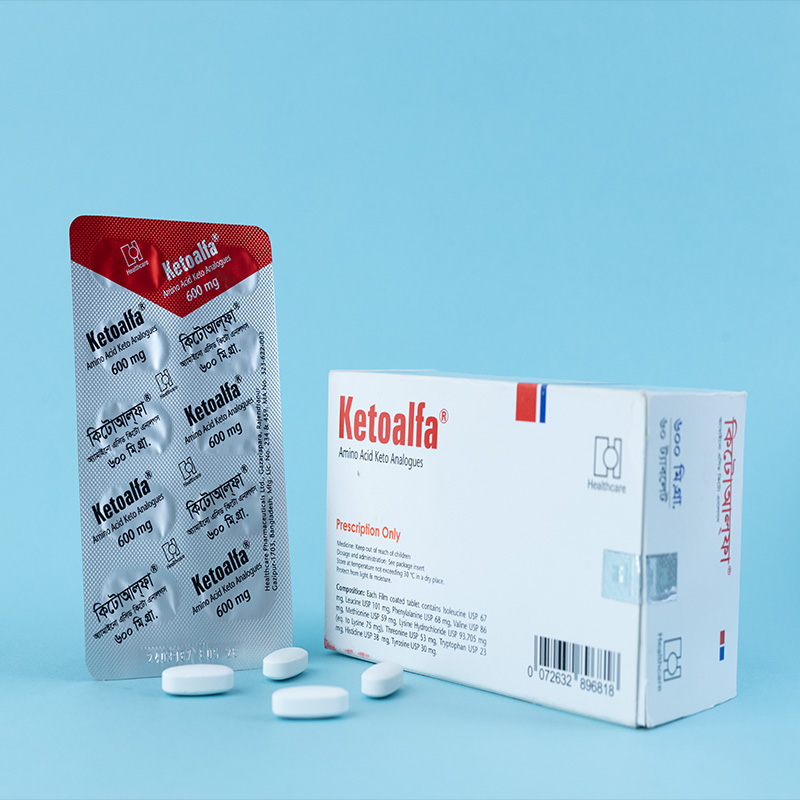
Ketoalfa is indicated for the prevention and treatment of damage caused by faulty or deficient protein metabolism in chronic kidney disease, in connection with a limited dietary protein intake of 40 g/day or less (for adults). This is typically applicable to patients whose glomerular filtration rate (GFR) is less than 25 ml/min.
Each film-coated tablet contains:
Calcium-3-methyl-2-oxo-valerate (α-ketoanalogue of isoleucine, calcium salt): 67 mg
Calcium-4-methyl-2-oxo-valerate (α-ketoanalogue of leucine, calcium salt): 101 mg
Calcium-2-oxo-3-phenylpropionate (α-ketoanalogue of phenylalanine, calcium salt): 68 mg
Calcium-3-methyl-2-oxobutyrate (α-ketoanalogue of valine, calcium salt): 86 mg
Calcium-DL-2-hydroxy-4-(methylthio) butyrate (α-hydroxyanalogue of methionine, calcium salt): 59 mg
L-lysine acetate USP (equivalent to Lysine 75 mg): 105 mg
L-threonine USP: 53 mg
L-tryptophan USP: 23 mg
L-histidine USP (as Histidine Hydrochloride Monohydrate): 38 mg
L-tyrosine USP: 30 mg
This nutritional supplement contains amino acids, partly in the form of their corresponding ketoanalogues, which are essential for patients with chronic kidney disease. It follows the same catabolic pathways as amino acids and works by improving protein metabolism in the body, thereby enhancing renal function. Amino acid ketoanalogues allow the intake of essential amino acids while minimizing nitrogen intake. The keto- and hydroxy-analogues are transaminated into the corresponding essential amino acids by utilizing nitrogen from non-essential amino acids. This process decreases urea formation by reusing the amino group and aids absorption.
Unless otherwise prescribed, the recommended dosage is 4–8 tablets, three times a day during meals. This dosage is applicable for adults with a body weight of approximately 70 kg.
Swallow the tablets whole. Do not chew.
The daily dietary protein intake should not exceed 40 g (for adults).
Ingestion during meals facilitates proper absorption and metabolism into the corresponding amino acids.
These tablets should be taken as long as the GFR remains between 5 and 25 mL/min.
Concomitant administration of calcium-containing drugs may increase serum calcium levels.
If aluminum hydroxide is taken, the dose of Ketoalfa should be reduced if necessary.
Drugs that form insoluble compounds with calcium (e.g., tetracyclines, quinolones such as ciprofloxacin and norfloxacin, and drugs containing iron, fluoride, or estramustine) should not be taken at the same time as Ketoalfa, to prevent impaired absorption of the active ingredients. Maintain a gap of at least two hours between taking these drugs and Ketoalfa.
The susceptibility to cardioactive glycosides increases, raising the risk of arrhythmia with Alpha ketoanalogue. Serum phosphate levels should be monitored in case of co-administration with aluminum hydroxide.
Ketoalfa is contraindicated in:
Hypersensitivity to the active ingredients or excipients.
Hypercalcemia.
Disturbed amino acid metabolism.
Very rare: Hypercalcemia.
If hypercalcemia occurs, the intake of vitamin D should be reduced.
If hypercalcemia persists, the dosage of Ketoalfa and any other calcium sources should be adjusted.
There are no adequate clinical data on the use of amino acid keto analogues in pregnant women.
Animal studies do not indicate direct or indirect harmful effects related to pregnancy, fetal development, labor, or postnatal growth.
Caution is advised when prescribing to pregnant women. There is no clinical experience regarding its use during lactation.
Regular monitoring of serum calcium levels is required.
Ensure adequate calorie intake while taking this supplement.
Patients with hereditary phenylketonuria should be cautious, as Ketoalfa contains phenylalanine.
Serum phosphate levels should be monitored when co-administered with aluminum hydroxide.
Store at a temperature not exceeding 30°C in a dry place.
Protect from light and moisture.