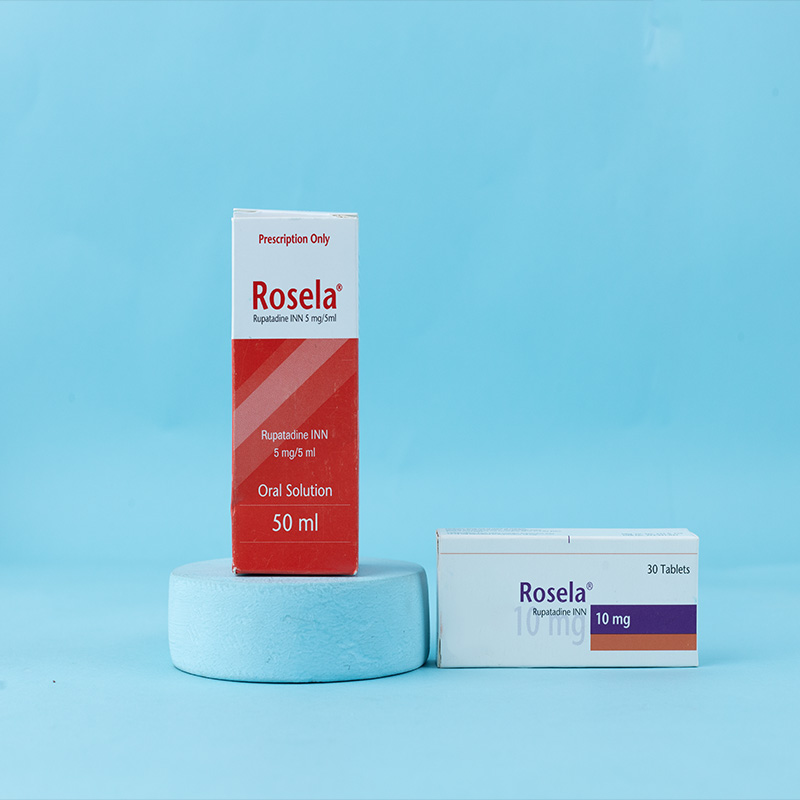
Zilas® Tablet 20 mg: Each tablet contains Bilastine INN 20 mg.
Zilas® is a non-sedative, long-acting histamine antagonist with selective peripheral H1 receptor activity and no affinity for muscarinic, serotonergic, or dopaminergic receptors. It has been developed for the symptomatic treatment of allergic rhinoconjunctivitis and urticaria. Zilas® works as an antiallergic agent by inhibiting immune system reactions mediated by the interaction of histamine on its H1 receptor. Studies conducted in vitro have demonstrated Bilastine’s high affinity and selectivity for the human H1 receptor.
Zilas® 20 mg tablet is indicated for the symptomatic treatment of allergic rhinoconjunctivitis and urticaria in adults.
The recommended dose of Zilas® is one tablet (20 mg) taken once daily for the relief of symptoms of allergic rhinoconjunctivitis (seasonal and perennial) and urticaria in adults. The tablet should be taken orally one hour before or two hours after consuming food or fruit juice.
Zilas® is contraindicated in patients with hypersensitivity to Bilastine or any of its ingredients. It is also contraindicated in individuals with a history of QT prolongation, including congenital long QT syndromes.
Zilas® should not be used in patients with a history of QTc prolongation, including congenital long QT syndromes. Caution is advised when administering Zilas® to patients with a history of cardiac arrhythmias, hypokalemia, hypomagnesemia, significant bradycardia, or a family history of sudden cardiac death.
No dosage adjustment is needed for patients with renal impairment.
Since Bilastine is not metabolized, hepatic impairment is not expected to increase systemic exposure beyond the safety margin. Therefore, no hepatic dosage adjustment is required.
The most common side effects of Zilas® are headache, drowsiness, and fatigue
There are no adequate and well-controlled studies on the use of Bilastine during pregnancy. It is advisable to avoid using Zilas® during pregnancy unless absolutely necessary.
The excretion of Bilastine in breast milk has not been studied in humans, and limited clinical data are available regarding its effect on fertility.
Children & Adolescents
The safety and efficacy of Zilas® in children under 12 years of age have not been established.
With Ketoconazole and Erythromycin: Concomitant administration of Bilastine 20 mg, Ketoconazole 400 mg once daily, and Erythromycin 500 mg three times daily increased Zilas® AUC 2-fold and Cmax 2-3 fold due to interaction with intestinal efflux transporters. However, these changes do not affect Zilas®'s safety profile.
With Diltiazem: Co-administration of Zilas® 20 mg once daily and Diltiazem 60 mg once daily increased the Cmax of Bilastine by 50%, but this does not appear to affect its safety.
With Alcohol: The psychomotor performance observed after concomitant intake of alcohol and Zilas® 20 mg was similar to that observed with alcohol and placebo.
With Lorazepam: Co-administration of Zilas® 20 mg once daily and Lorazepam 3 mg once daily for 8 days did not enhance the CNS depressant effects of Lorazepam.
In cases of overdose, the frequency of treatment-emergent adverse events was approximately twice that of placebo. The most commonly reported adverse effects were dizziness, headache, and nausea. Symptomatic and supportive treatment is recommended in such cases.
Store at a temperature not exceeding 30°C in a dry place. Protect from light and moisture.