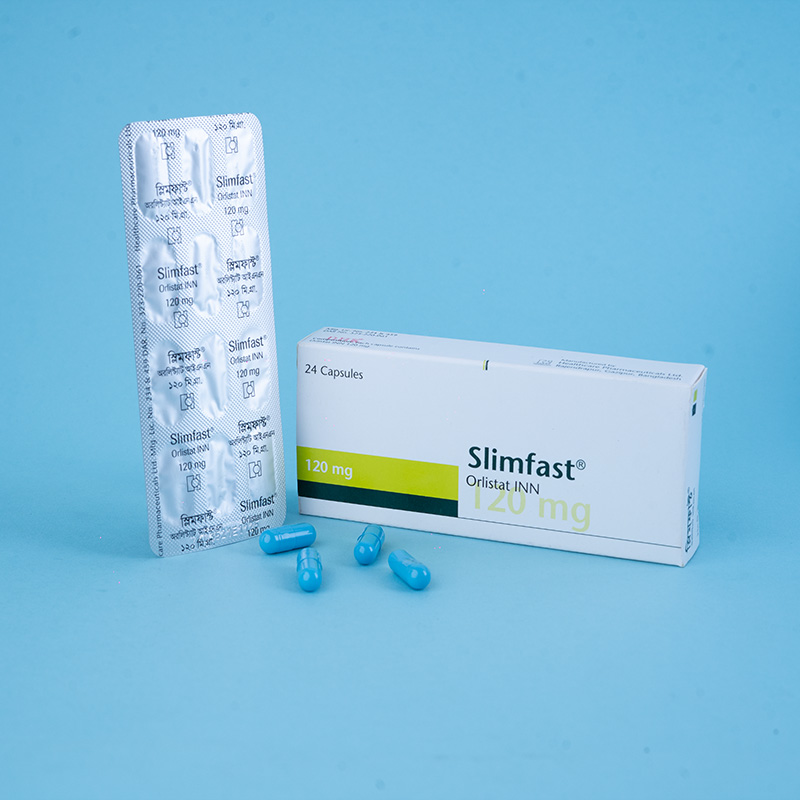
Each capsule contains Orlistat INN 60 mg as the active ingredient.
Orlistat is a potent, specific, and long-acting lipase inhibitor. It works in the stomach and upper small intestine by forming a covalent bond with the active serine site of gastric and pancreatic lipases. The inactivated enzyme cannot hydrolyze dietary fats (triglycerides) into absorbable free fatty acids and monoglycerides. As a result, undigested triglycerides are not absorbed, creating a caloric deficit that supports weight control.
Systemic absorption of orlistat is not necessary for its activity. At the recommended therapeutic dose of 60 mg three times daily, Orlistat inhibits dietary fat absorption by approximately 30%.
Adults:
Orlistat is indicated for use alongside a mildly hypocaloric diet for:
Obese patients with a body mass index (BMI) ≥30 kg/m².
Overweight patients (BMI ≥28 kg/m²) with associated risk factors such as type II diabetes, hyperlipidemia, or hypertension.
Treatment should be discontinued if patients fail to lose at least 5% of their body weight within 12 weeks of starting therapy.
Adolescents (12 years and older):
Orlistat may be prescribed for obese adolescents when weight reduction cannot be achieved through diet and physical activity. It is especially beneficial if obesity-related complications are present.
Recommended Dose: One 60 mg capsule taken immediately before, during, or up to one hour after each main meal.
If a meal is missed or contains no fat, skip the dose of Orlistat.
Doses exceeding 60 mg three times daily provide no additional benefits.
The therapeutic effects include an increase in fecal fat within 24-48 hours of dosing. Upon discontinuation, fecal fat content usually returns to pretreatment levels within 48-72 hours.
The tolerability and efficacy of Orlistat have not been studied in elderly patients or those with hepatic or renal impairments.
Orlistat is contraindicated in patients with:
Chronic malabsorption syndrome.
Cholestasis.
Hypersensitivity to Orlistat or its ingredients.
Weight reduction with Orlistat may be less significant in type II diabetic patients. Monitor antidiabetic medication closely, as glycemic control improvement may require dosage adjustments for oral antidiabetics or insulin.
Patients must adhere to dietary recommendations, as gastrointestinal side effects are more likely with high-fat meals. Daily fat intake should be distributed across three main meals.
Multivitamin supplements containing fat-soluble vitamins (A, D, E, K) should be taken to ensure proper nutrition, as Orlistat can reduce the absorption of these vitamins and beta-carotene.
The most common side effects are gastrointestinal and may include:
Oily spotting from the rectum.
Flatulence.
Fecal urgency.
Oily or fatty stools.
Abdominal discomfort and frequent bowel movements.
Rare side effects may include:
Influenza-like symptoms.
Rectal bleeding, diverticulitis, pancreatitis, or gallstones.
Hepatitis with symptoms like jaundice, itching, or stomach pain.
Hypersensitivity reactions such as rash, angioedema, or anaphylaxis.
No interactions have been observed with commonly prescribed drugs, including alcohol, digoxin, nifedipine, oral contraceptives, phenytoin, pravastatin, warfarin, or metformin.
Avoid concurrent use with other fat-soluble medications unless directed by a healthcare provider.
Orlistat should not be used during pregnancy due to a lack of clinical data.
It is unknown whether Orlistat is excreted in breast milk, so its use is not recommended while breastfeeding.
Store below 30°C in a dry place.
Protect from light and moisture.
Keep out of reach of children.