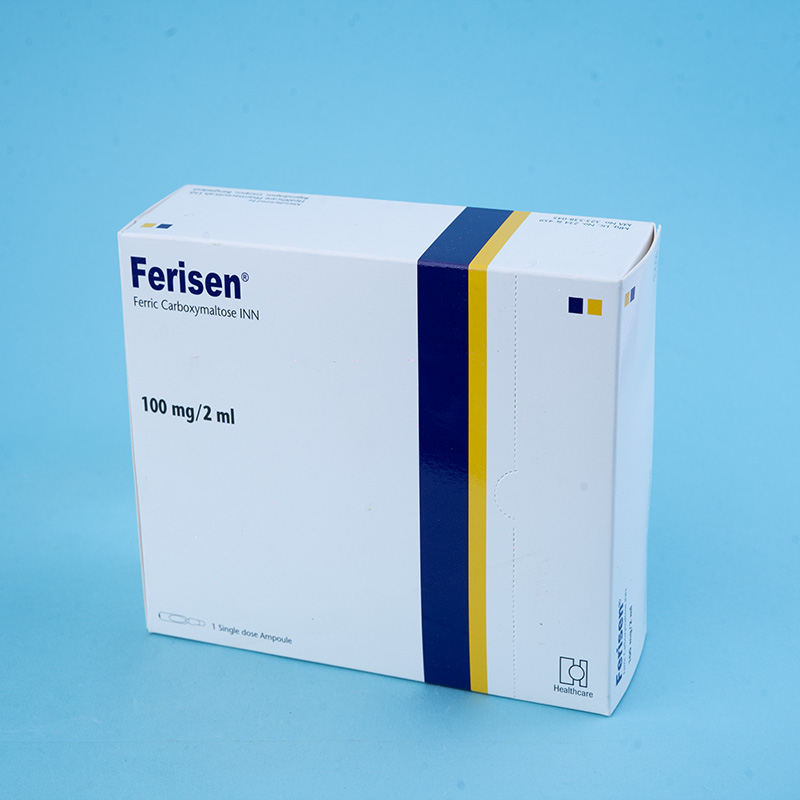
Ferisen® 100 mg/2 ml Injection: Each 2 ml injection contains 322.58 mg of Ferric Carboxymaltose INN, equivalent to 100 mg of iron.
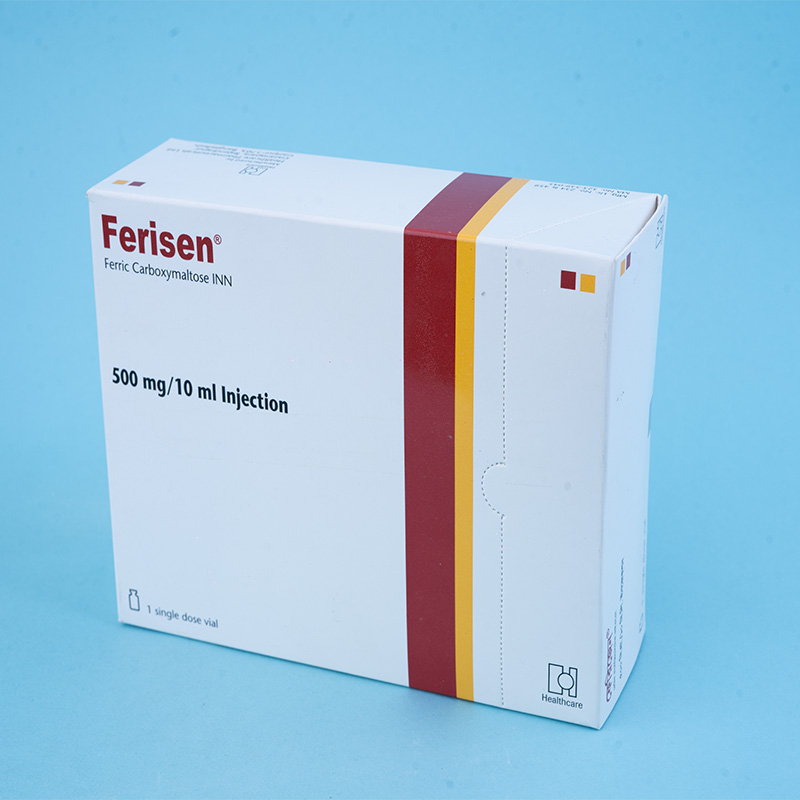
Ferisen® 500 mg/10 ml Injection: Each 10 ml injection contains 1612.90 mg of Ferric Carboxymaltose INN, equivalent to 500 mg of iron.
Ferric carboxymaltose is an iron replacement product indicated for the treatment of iron deficiency anemia in adult patients:
Who have an intolerance to oral iron or have had an unsatisfactory response to oral iron.
Who have non-dialysis-dependent chronic kidney disease.
The diagnosis of iron deficiency must be based on laboratory tests.
Posology
The dosage of ferric carboxymaltose follows a stepwise approach:
Step 1: Determination of the individual iron need.
Step 2: Calculation and administration of the iron dose(s).
Step 3: Post-iron repletion assessments.
These steps are outlined below:
Step 1: Determination of the Iron Need
The individual iron requirement for repletion using ferric carboxymaltose is determined based on the patient's body weight and hemoglobin (Hb) level. Refer to Table 1 for details.
Step 2: Calculation and Administration of the Maximum Individual Iron Dose(s)
Based on the iron need determined above, the appropriate dose(s) of ferric carboxymaltose should be administered, considering the following:
A single ferric carboxymaltose administration should not exceed:
15 mg iron/kg body weight (for administration by intravenous injection).
20 mg iron/kg body weight (for administration by intravenous infusion).
1,000 mg of iron (20 ml ferric carboxymaltose).
The maximum recommended cumulative dose of ferric carboxymaltose is 1,000 mg of iron (20 ml ferric carboxymaltose) per week.
Step 3: Post-Iron Repletion Assessments
Reassessment should be performed by the clinician based on the individual patient's condition. The Hb level should be reassessed no earlier than 4 weeks post-final ferric carboxymaltose administration to allow adequate time for erythropoiesis and iron utilization.
a. Patients with Hemodialysis-Dependent Chronic Kidney Disease
A single maximum daily injection dose of 200 mg iron should not be exceeded in hemodialysis-dependent chronic kidney disease patients.
Ferric carboxymaltose must only be administered via the intravenous route:
By injection
By infusion
During a hemodialysis session (undiluted, directly into the venous limb of the dialyzer).
Ferric carboxymaltose must NOT be administered subcutaneously or intramuscularly.
a) Intravenous Injection
Ferric carboxymaltose may be administered undiluted via intravenous injection. The maximum single dose is 15 mg iron/kg body weight, but should not exceed 1,000 mg iron.
b) Intravenous Infusion
Ferric carboxymaltose may be administered via intravenous infusion but must be diluted.
The maximum single dose is 20 mg iron/kg body weight, but should not exceed 1,000 mg iron.
It must be diluted only in sterile 0.9% m/V sodium chloride solution, as per Table 3.
For stability reasons, ferric carboxymaltose should not be diluted to concentrations below 2 mg iron/ml.
Ferric carboxymaltose is contraindicated in patients with:
Hypersensitivity to the active substance, ferric carboxymaltose, or any excipients.
Serious hypersensitivity to other parenteral iron products.
Anemia not caused by iron deficiency (e.g., other microcytic anemia).
Evidence of iron overload or disturbances in iron utilization.
a. Pregnancy
Pregnancy Category: C.
There are no adequate and well-controlled trials in pregnant women.
Ferric carboxymaltose should only be used if clearly necessary, following a careful benefit-risk evaluation.
b. Breastfeeding
Clinical studies show that transfer of iron from ferric carboxymaltose to breast milk is negligible (≤1%).
Based on limited data, it is unlikely to pose a risk to the breastfed child.
c. Fertility
No human fertility data are available.
Animal studies indicate that fertility was unaffected by ferric carboxymaltose treatment.
The most common adverse reactions (≥2%) include:
Nausea
Hypertension
Flushing
Hypophosphatemia
Dizziness
Overdose
Excessive administration of ferric carboxymaltose may cause iron accumulation in storage sites, leading to hemosiderosis.
Monitoring iron parameters (serum ferritin and transferrin saturation) can help detect iron accumulation. If necessary, treatment may include iron chelation therapy.
Special Precautions for Disposal and Handling
Inspect ampoules for sediment and damage before use.
Use only sediment-free, homogeneous solutions.
Each ampoule is for single use only. Dispose of unused products according to local regulations.
Ferric carboxymaltose must only be mixed with sterile 0.9% sodium chloride solution.
Do not mix with other intravenous solutions or therapeutic agents (risk of precipitation or interaction).
Store at a temperature not exceeding 30°C in a dry place.
Protect from light.
Do not freeze.
Keep out of reach of children.